Blog Articles
Addressing the Lack of Capacity in Drug Production: How Ocyonbio in Puerto Rico is Stepping Up
In recent years, the pharmaceutical industry has been facing a growing issue of lack of capacity in drug production, particularly when it comes to sterile […]
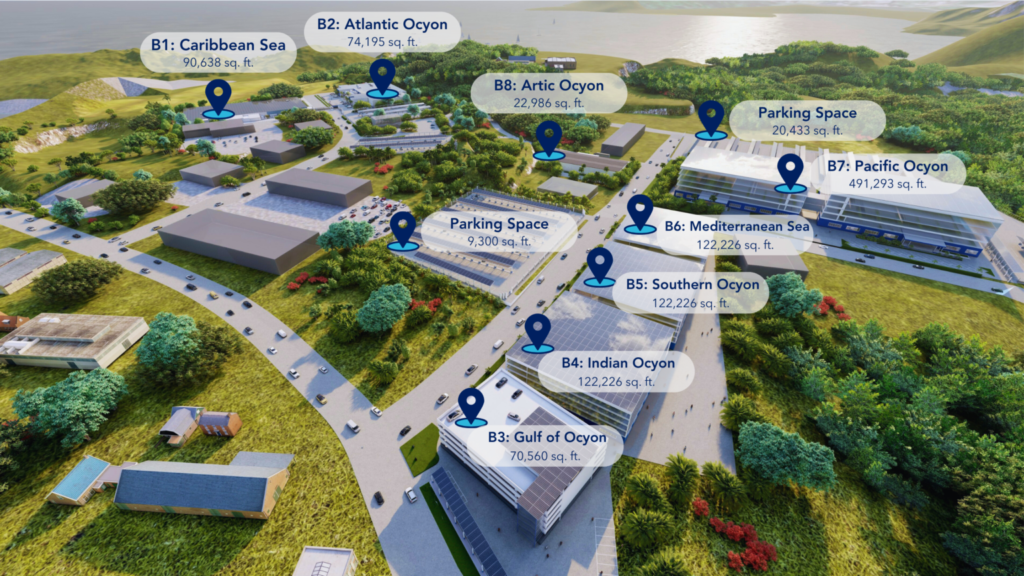
OcyonBio’s Campus Expansion on Puerto Rico
As OcyonBio develops itself to help Puerto Rico become an innovative pharma hub for the United States, we plan to significantly expand the facility space […]
Accelerate Your Time to Clinic with OcyonBio
In the fast-paced world of pharmaceutical manufacturing, time is a critical factor. The sooner a product can be brought to the clinic, the sooner it […]
Discover the Unique Value Proposition of OcyonBio
OcyonBio stands at the forefront of pharmaceutical manufacturing, offering a comprehensive suite of services designed to meet the needs of companies looking to expand their […]
BIO International Convention 2024
OcyonBio will be attending the BIO International Convention 2024, the largest and most comprehensive event for biotechnology. Please join us at the event from June […]
OCYONBIO to Showcase Cutting-Edge Capabilities at BIO International Conference in San Diego
OCYONBIO, a leader in the pharmaceutical manufacturing sector, is gearing up to make a significant impact at the upcoming Bio International Conference, scheduled for June […]
Puerto Rico’s Bioscience Highlight: OCYONBIO’s Strategic Expansion and the Island’s STEM Legacy
The pharmaceutical industry’s challenge with the shortage of sterile injectable capacity underscores the importance of strategic expansion and innovation in manufacturing capabilities. OCYONBIO’s initiative to […]
Addressing the Sterile Injectable Capacity Shortage: OCYONBIO’s Strategic Expansion and Puerto Rican Incentives
The pharmaceutical industry faces a significant challenge in the form of a shortage of sterile injectable capacity. This bottleneck has implications for the availability of […]
Enabling Pharmaceutical Innovation in Puerto Rico with OcyonBio
In the fast-paced world of pharmaceutical development, the focus often shifts towards the next big discovery or technological breakthrough. However, for OcyonBio, a leading Partnership […]
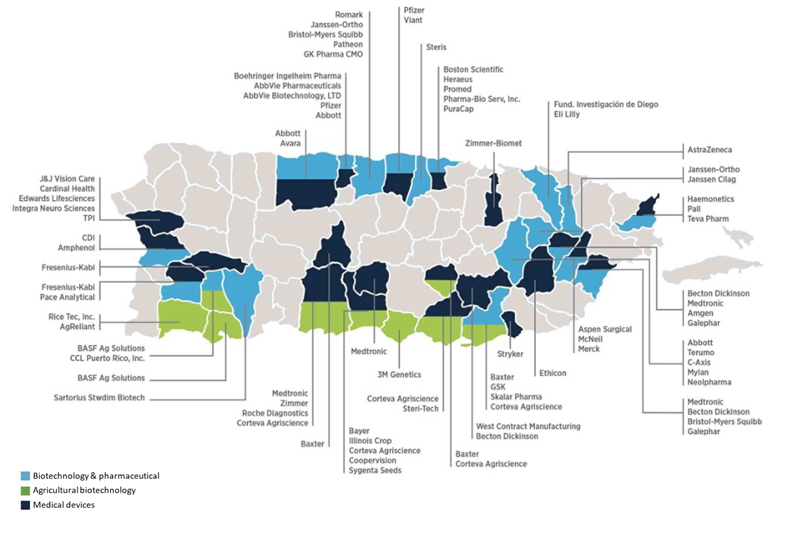
Puerto Rico’s Robust Infrastructure and Collaborative Ecosystem: A Haven for Pharmaceutical Companies
The beautiful island of Puerto Rico is not only a tropical paradise but has also emerged as a thriving hub for numerous industries, including pharmaceuticals. […]
Empowering Business Growth in Puerto Rico with OcyonBio’s OcyonEleven Campus
In Puerto Rico, there is a company with a mission to revolutionize the pharmaceutical industry and foster economic growth on the island. That company is […]
Unlocking Economic Opportunities in Puerto Rico
Puerto Rico, a vibrant island in the Caribbean, is not only known for its breathtaking landscapes and rich culture but also for the array of […]
Strategic Partnerships in Pharmaceutical Manufacturing: Identifying the Need for a PDMO Collaboration
In the pharmaceutical industry, the concept of a Partnership Development Manufacturing Organization (PDMO) represents a collaborative approach to drug product development and manufacturing. This partnership […]
Navigating the Complexities of Sterile Filling in Pharmaceuticals: The Strategic Role of PDMOs
In the pharmaceutical industry, sterile filling is a cornerstone of drug manufacturing, ensuring that products are safe and effective for consumer use. This critical step […]
Unveiling the Beneficial Long-term Tax Incentives for Pharmaceutical Businesses in Puerto Rico
The picturesque island of Puerto Rico has been continuously fostering a business-friendly environment, enticing various industries to establish their roots on its shores. Among the […]
C.P.H.I. India – Why OcyonBio and Puerto Rico?
On the week of November 27th, OcyonBio and Invest Puerto Rico were both present at the Convention on Pharmaceutical Ingredients (CPhI) to explain the benefits […]
The Allure of Puerto Rico: A Prime Destination for Pharmaceutical Ventures
Puerto Rico, with its rich history, vibrant culture, and stunning landscapes, is a tourist paradise and a thriving hub for the pharmaceutical industry. The island […]
OcyonBio and InvestPR at C.P.H.I. India
OcyonBio was at C.P.H.I. India to speak alongside InvestPR to discuss how Puerto Rico, with its sixty years of pharmaceutical history; and OcyonBio as a […]
Unlocking the Potential of Cell and Gene Therapy: A Revolutionary Approach to Treating Genetic Disorders
Cell and gene therapies are revolutionary approaches in the field of medicine that aim to treat, prevent, or potentially cure diseases by addressing the underlying […]
Fostering a Culture of Innovation and Excellence: The Role of OcyonBio and Similar Pharmaceutical Companies in Puerto Rico and Beyond
The pharmaceutical industry is a dynamic and ever-evolving field that plays a crucial role in improving public health. In Puerto Rico, companies like OcyonBio are […]
Rethinking Regulatory Compliance: Beyond the FDA Mandated Consultants
In the intricate complexity of pharmaceutical manufacturing, regulatory compliance emerges as a pivotal thread, particularly in the context of accessing the lucrative US market. The […]
Unleashing the Future of Cancer Treatment: Key Therapeutic Breakthroughs in 2023
In recent years, scientific advancements in the field of oncology have given hope to millions of people around the world. With cancer being one of […]
Optimizing Vial Fill and Finish Capacity with OcyonBio: A Guide to Efficient and High-Quality Pharmaceutical Production
The vial fill and finish process is a critical stage in the pharmaceutical manufacturing landscape. Not only does it require precision and accuracy, but it […]
Unlocking the Potential: How Puerto Rico Incentives Empower and Benefit Pharmaceutical Businesses
Puerto Rico has long been a hub for pharmaceutical companies, with its strategic location, skilled workforce, and attractive tax incentives. These incentives have helped pharmaceutical […]
PRBio Tech Hub: Driving Innovation and Economic Growth in Puerto Rico
The Biden-Harris Administration’s commitment to fostering technological innovation and job creation across the United States has reached a significant milestone. In a groundbreaking move, […]
Discover the Thriving Ocyon Eleven Campus: Affordable Solutions for Business Growth
Choosing the right location for your business is crucial for success. Ocyon Eleven Campus, an innovative technological hub, offers a unique blend of affordability, sustainability, […]
Uncover the Benefits of OcyonBio’s CDMO Solutions for cGMP Fill and Finish Services and Optimize Your Manufacturing Process
In the fast-growing pharmaceutical industry, drug developers and manufacturers face numerous challenges that can impede the timely and cost-effective delivery of life-saving medications to patients. […]
Ocyon Eleven Campus: Affordable Leasing Solutions for Biotech Companies
Ocyon Eleven Campus: The Ideal Leasing Solution for Biotech Companies In the rapidly evolving world of biotechnology, having a conducive environment to foster innovation […]
Ocyon Eleven: Revolutionizing Biologics in Puerto Rico
Welcome to the vibrant world of Ocyon Eleven, a biologics company making waves in Puerto Rico. With cutting-edge research and state-of-the-art facilities, Ocyon Eleven transforms […]
The Future of Cell Therapy: Trends to Watch in 2023
At OcyonBio, we are thrilled to see that patients with rare diseases have a brighter outlook than ever before. The advancements in cell therapy have [...]
Revolutionize Your Biotech Process Development Operations with OcyonBio’s Expertise
OcyonBio is a leading provider of cell and gene therapy manufacturing services. Our team of experts has decades of experience in the biotech industry, and [...]
Solutions for the Challenges of Maintaining Viral Vector Stability & Quality through Process Development
Viral vectors, which acts as containers of a cell’s modified genetic material, were first used in research to transfer or alter genetic material in cells. [...]
Partnership Development Management Organization(PDMO) – CDMO alternative for Gene and Cell Therapy, Virus and Biologics Mfg.
PDMO – Partnership Development Management Organization The following is simplistic introduction to a CDMO alternative. This new PDMO concept is to enable the same capability [...]
Donor Derived Supply Clift Risk(s)- Gene and Cell Therapy Companies
As of this writing there are more than 40 companies using donor-derived cells to manufacture their intended version of cell-based therapies. Further a review of [...]
OCYONBIO cGMP space and Viral Manufacturing Capabilities Ready to Go !
Happy New Year to our OCYONBIO team, THANK YOU for the extra ordinary 2021. In 2022, our goal is enabling our partnership development organization campus [...]
Non-Dilutive Funding driven by Business Incentive Office (BIO) by OCYONBIO
Introduction to Business Incentive Optimization by OCYONBIO Business Incentive Optimization office was created to help companies establish their business in Puerto Rico. This office was [...]