Our Model
Ocyonbio helps you save money while advancing your pipeline

Shared Resources to lower overall cost
Side by Side Technical Transfer to protect your IP
Full control of your space and schedule
“BIO” Support you on maximizing grants and credits
Solving the Start-up Paradox
Go fast with Minimal Spending
Typical Start-up Problems we solve:
- Promising pipeline – No places to develop CMC
- Need to go fast with limited funding
- Need cGMP infrastructure to advance through process development stages
- CDMO cost for first product consumes all capital
- CDMO timelines not feasible
- Need to fund second and third product
- Cash and Speed not aligned
- Need to non-dilutive investment
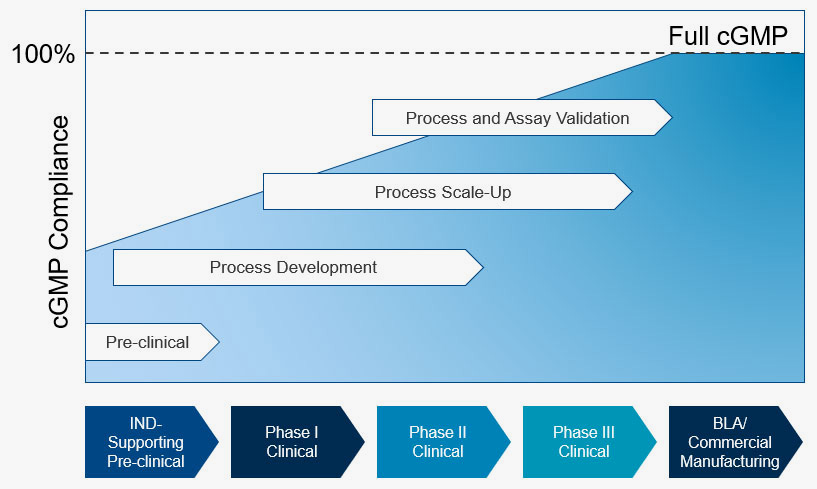
Supportive Process for every phase of the product development spectrum
Full ECOSYSTEM in ONE Campus
R & D
Manufacturing
Manufacturing Space available for early and pre-clinical stage companies
Clinical
Manufacturing
ISO7 Clean Rooms equipped for immediate technical transfer from university, hospitals and early R & D
cGMP
Manufacturing
Large manufacturing suites design for late stage clinical and commercialization
Viral and
Cell Processing
Lenti, Retroviral and AAV produced with closed systems in cGMP ISO 7 facility
Cord Blood and Leukapheresis cell separation supporting NK, T, B and TREG banks
K562 Feeder Cells
Laboratory
Release Testing
State of the Art Laboratory supporting potency assays, Flow, PCR, sterility, and required release testing
OcyonBio Advantage Puerto Rico
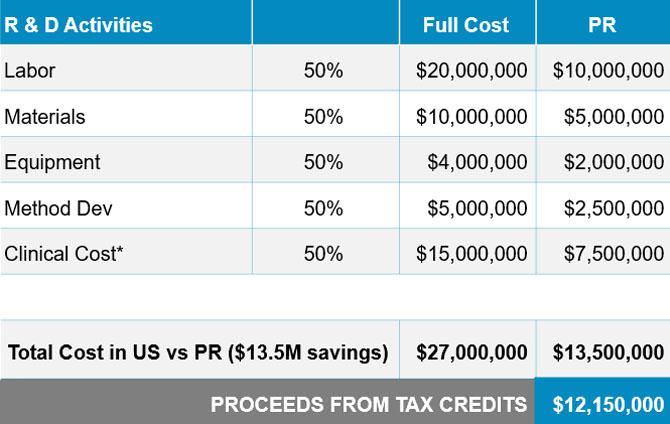
Offers 50% R & D Sellable Tax Credits
- Puerto Rico offers 50% Tax Credit that are immediately sellable
- All R & D activities receive a 50% subsidy – People, materials, equipment, clinical and other expenses
- Availability of high skill resources ( Amgen, AbbVie, Cytoimmune, Cytovia)
- US Quality Manufacturing with FDA regulatory Process
- Facility Customizable to suit Partners’ manufacturing needs
- Access to (T,B,NK cells)
- Hotelling offering with customized build-out
Business Optimization Incentive Office (BIO)
To Expand Your Money