
Uncover the Benefits of OcyonBio’s CDMO Solutions for cGMP Fill and Finish Services and Optimize Your Manufacturing Process
In the fast-growing pharmaceutical industry, drug developers and manufacturers face numerous challenges that can impede the timely and cost-effective delivery of life-saving medications to patients. One critical aspect of the production process that can significantly impact the efficiency and quality of pharmaceutical products is fill and finish services. OcyonBio, a Contract Development and Manufacturing Organization (CDMO), offers cutting-edge solutions for cGMP fill and finish services. In this article, we will explore how partnering with OcyonBio can help pharmaceutical companies optimize their manufacturing processes and unlock a range of benefits.

1. Superior Expertise and State-of-the-Art Facility:
OcyonBio stands out as a trusted partner for cGMP fill and finish services due to its team of highly skilled experts and soon-to-be advanced manufacturing facility. The company boasts a dedicated team experienced in handling complex filling processes, ensuring precision, speed, and compliance with regulatory requirements. Additionally, OcyonBio’s facility will be equipped with state-of-the-art technology from Marchesini Group USA and adhere to the highest quality standards, enabling a streamlined and efficient manufacturing process.
2. Customizable Solutions:
OcyonBio recognizes that each drug formulation and production process is unique. Therefore, they offer customizable fill and finish solutions tailored to meet the specific needs of their clients. By working closely with pharmaceutical companies, OcyonBio can adapt their services to accommodate different dosage forms, container types, and batch sizes. This flexibility not only ensures the efficient delivery of products but also minimizes waste and optimizes resource utilization.
3. Accelerated Time-to-Market:
In the competitive pharmaceutical landscape, getting products to market quickly is crucial. OcyonBio’s expertise in fill and finish services helps to expedite the manufacturing timeline. Their streamlined processes, comprehensive quality control measures, and experienced team enable a faster turnaround time, reducing overall project timelines and accelerating time-to-market.
4. Regulatory Compliance:
Ensuring compliance with Good Manufacturing Practices (GMP) regulations is paramount in the pharmaceutical industry. OcyonBio’s future GMP-compliant facility and rigorous quality control systems ensure that all fill and finish services adhere to regulatory guidelines. By partnering with OcyonBio, pharmaceutical companies can have peace of mind, knowing that their products meet the highest quality standards and comply with regulatory requirements.
5. Cost-Effective Solutions:
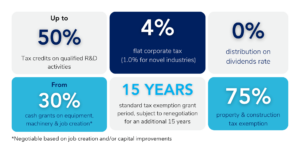
Outsourcing fill and finish services to OcyonBio can provide notable cost advantages for pharmaceutical companies. By partnering with a CDMO like OcyonBio, pharmaceutical companies can avoid or minimize the need for additional infrastructure investment, saving capital for other critical areas of drug development. Moreover, by establishing your operations in Puerto Rico you can leverage Puerto Rico R&D tax credits to obtain up 50% in annual cash refund of monies spent and reduce up to 20% of your annual spend outside of the island.
OcyonBio’s CDMO solutions for cGMP fill and finish services offer pharmaceutical companies a range of benefits, from superior expertise in the field and customizable solutions. By partnering with OcyonBio, companies can optimize their manufacturing processes, accelerate time-to-market, ensure regulatory compliance, and achieve cost savings. As the pharmaceutical industry continues to evolve, collaborating with a trusted CDMO like OcyonBio can provide the competitive edge necessary for success in the marketplace.

