Press Releases
OCYONBIO Announces Strategic Agreement with Marchesini Group USA for the Purchase of Filling and Packaging Equipment, Providing Vital Capabilities for Clients
Agreement brings CDMO Capabilities to OCYONBIO to Support Clients Sterile Filling Requirements and Provide cGMP Packaging and Inspection FROM: GlobeNewswire OCYONBIO, a contract development and manufacturing […]
Talento e infraestructura son prioridades para las compañías de biociencias
Ella Woger-Nieves, principal oficial ejecutiva interina de Invest Puerto Rico, busca que Puerto Rico se convierta en una destino de calibre mundial en este sector. [...]
OcyonBio to hold its ‘Gene & Cell Therapy’ webinar June 16
OcyonBio, a firm that researches and develops cell and genetic treatments, will host the Gene & Cell Therapy Webinar event on June 16 from 4 [...]
OcyonBio to Offer Webinar to Local Scientists and Researchers
The event is oriented toward professionals interested in better understanding the company’s operations on the island. Source: The Weekly Journal https://www.theweeklyjournal.com/business/ocyonbio-to-offer-webinar-to-local-scientists-and-researchers/article_476ae19a-ec10-11ec-a441-677395abb533.html OcyonBio, a company dedicated [...]
New scientific research and development incubator in Aguadilla
There are only five other gene and cell therapy hubs in the U.S. - Juan A. Hernández, The Weekly Journal Source: El Vocero https://issuu.com/vocero.com/docs/wj06082022/7 OcyonBio, [...]
Invest Puerto Rico seeks to attract bioscience and technology companies
Its 'Game Changers, Welcome Home' campaign seeks to position Puerto Rico as a global center of innovation Fuente: Primera Hora https://www.primerahora.com/brandstudio/todos-por-puerto-rico/notas/invest-puerto-rico-busca-atraer-empresas-de-biociencias-y-tecnologia This content was [...]
“Ocyonbio” recluta personal para empresa de investigación y manufactura de medicamentos biosimilares
La empresa combinará una fábrica de medicamentos biosimilares y la primera incubadora de investigación científica con miras a desarrollar la cura del cáncer y el [...]
Empresa OcyonBio invierte $158 millones en su avanzada sede en Aguadilla
Crea un centro de laboratorios para investigación y desarrollo de tratamientos celulares contra distintos tipos de cáncer y otras enfermedades Fuente: El Nuevo Día https://www.elnuevodia.com/negocios/empresas-comercios/notas/empresa-ocyonbio-invierte-158-millones-en-su-avanzada-sede-en-aguadilla/?r=79121 [...]
Más de $80,000 el salario promedio en la industria biofarmacéutica
Estudio comisionado por PIA le atribuye a este sector de la manufactura más de $10,000 millones de inversión, 37% de los ingresos del fisco y [...]
Biosimilar Solutions Initiates Registrational Clinical Trial for Biosimilar Version Neulasta OcyonBio
Biosimilar Solutions, Inc. will set up a facility in Aguadilla in OcyonBio's Campus in Puerto Rico to support the production of BSC0826. Featured Image for [...]
OcyonBio Client Biosimilar Solutions, Inc. Initiates Registrational Clinical Trial for Its First Biosimilar Product
Biosimilar Solutions, Inc. expects to complete the clinical study in 2022 AGUADILLA, Puerto Rico, March 23, 2022 (GLOBE NEWSWIRE) -- Biosimilar Solutions, Inc. announces it [...]
OcyonBio Announces Biosimilar Solutions to Begin GMP Manufacturing in Puerto Rico
AGUADILLA, Puerto Rico, (Newswire.com) - OcyonBio announces manufacturing and operations agreement to develop biosimilar drug product facilities for Biosimilar Solutions, Inc. OcyonBio is creating an [...]
OcyonBio Appoints Ricardo Zayas as Chief Operating Officer
Experienced operational executive to lead OcyonBio through its next stage of growth AGUADILLA, Puerto Rico, February 14, 2022 (Newswire.com) - OcyonBio, LLC announces it has [...]
OcyonBio Appoints Daniel Chang, CPA, MBA, CPMP, CPIM as Chief Financial Officer
Daniel Chang AGUADILLA, Puerto Rico, Jan. 25, 2022 (GLOBE NEWSWIRE) -- OcyonBio, LLC announces it has appointed Daniel Chang Chief Financial Officer. OcyonBio is creating [...]
Health Tech: Robert Salcedo On How OcyonBio’s Technology Can Make An Important Impact On Our Overall Wellness
Find something you love and pursue it with all your might, be prepared to stumble and let the journey be what defines success. Any financial [...]
OcyonBio Appoints Robert Salcedo as Chief Executive Officer
AGUADILLA, Puerto Rico, Jan. 06, 2022 (GLOBE NEWSWIRE) -- OcyonBio, LLC is creating cell and gene therapy partnership development and manufacturing organization (PDMO). OcyonBio will [...]
Biosimilar Solutions, Inc. enters License and Development Agreement with Reliance Life Sciences Ltd
Biosimilar Solutions Inc., (“BSS”) a leading biosimilar company that develops and commercializes high-quality therapeutics for major regulated markets, announced that it has entered a license [...]
Invest Puerto Rico elevates the Island’s role as a global bioscience R&D and manufacturing hub attracting two major life-critical investments, $228M in new activity
– CytoImmune Therapeutics and Biosimilar Sciences US will soon call Puerto Rico home and engage in cancer immunotherapy solutions and a COVID-19 vaccine, respectively – [...]
Corona Virus – Real World Experience Directly from Front Lines
Storm before the Calm – Lessons Learned from Largest Biologic Contract Development and Manufacturing Company in China Written with Direct Permission from Wuxi Biologics China [...]
Regulatory Convergence -2020 Perspective
2020 Regulatory Convergence Pays Off The following article briefly walks through the current state of regulatory convergence that is occurring at both countries and company [...]
Top 10 Takeaways to New about New Biosimilar Interchangeability Guidance
The long-awaited formal guidance for interchangeability has been issued by US FDA. Once again, the core resounding message is the need for scientifically sound analytical [...]
Biosimilar Action Plan Long Way to Go
The FDA released the biosimilar action plan July 2018 and held meetings in early September 2018 to obtain input from public. In the September meeting [...]
Game Changer – FDA Biosimilar Action Plan
The FDA has announced the creation of Biosimilar Action Plan. The aim of this plan is to clarify and increase efficiencies in the Biosimilar Approval [...]
Clinical Lessons Learn – Biosimilar Programs
In biosimilar product development, strategic planning at the outset is critical in order to adequately manage planned resources and ensure timely entry of biosimilar products [...]
Analytical Similarity Gaps and Lessons Learned
Demonstration of Analytical Similarity for Global Biosimilar Applications - Part 2 of 3 Establishing analytical similarity is the core of any biosimilar program. The FDA [...]
Early Considerations for Global Biosimilar Application – Part 1 of 3
Background The following post will review some of the key gaps that often find when a sponsor ask us to evaluate their products for EU [...]
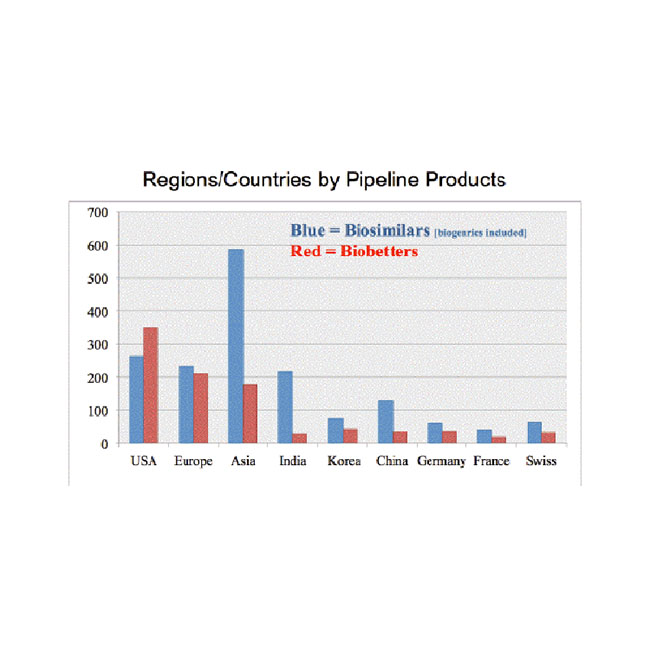
Biosimilar Development – Big Boys Game
As part of my role in my company I'm constantly reading about the many companies who are entering the biosimilar race. An interesting question raised [...]