Company Overview
OcyonBio provides dedicated autonomous manufacturing capacity with interconnected infrastructure and systems to support phased appropriate development for early development, pre-clinical, clinical, and commercial start. We behave more like a cGMP incubator space with all regulatory, systems capabilities, and resources to enable CMC data to support regulatory applications. Manufacturing and development spaces are designed to be autonomous while being interconnected to systems required to support clinical and commercial requirements.
OcyonBio provides a company with its own space, so there is no need to build an expensive facility. Providing flexibility to protect IP, manage schedules, resources, and new product introduction reducing overall cost and risks.

Disruptive Model
Accelerate from early clinical to commercial by providing facility, people and systems.

Cell Therapies
OcyonBio is a partnership development and manufacturing organization focused on advancing gene and cell therapies.

Own your facility
Enables facility ownership which allows for greater control over the process.

Shared Infrastructure
R+D suites, cell and gene therapy suites, virus and cell processing capabilities.

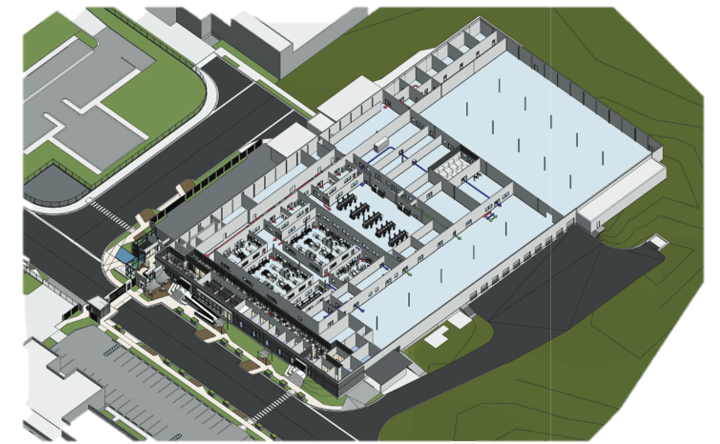
Facilities
Two buildings with over 150,000 square feet of space, cell processing capabilities in Q1, 2022, viral production capability in Q2, 2022.
Experienced Scientific and Commercial Leadership
Robert Salcedo
CO-Founder & Chief Executive Officer

Robert Salcedo
CO-Founder & CEO
Robert Salcedo is the CEO of OcyonBio Gene and Cell Therapy Partnership Development Organization. He is also the CEO and co-founder of Biosimilar Solutions and Biosciences Corp LLC. Robert Salcedo has 25+ years of experience in Biotechnology and Solid Dose Manufacturing experience, which includes 10+ years of working closely with start-ups in gene and cell therapy companies. He has led executive teams in developing Regulatory, CMC, Clinical, and manufacturing scale-up strategies and constructing commercial facilities ranging from $20MM to $1.5Bn. He was a leader in Amgen’s construction and technology transfer in Puerto Rico, which supplies drug products to the world. He previously consulted and advised 50+ clients focused on both gene and cell therapy and biologics companies.
Professional Experience:

Commercialization Experience:

Daniel Chang
CO-Founder & Chief Financial Officer

Daniel Chang
CPA, MBA, CPIM, CIRM, cPMP
CO-Founder, Chief Financial Officer
Daniel Chang is the Chief Financial Officer of OcyonBio. He is also the CFO and co-founder of Biosimilar Solutions and Biosciences Corp LLC. He has 20 years’ experience in Bio-Pharma and biosimilars as an executive. He has led product development for 30+ INDs.
Professional Experience:

Commercialization Experience:

Our Management Team
Alexandra Ortiz Rosa, MS
Director of Cell & Gene Therapy Unit

Alexandra Ortiz Rosa, MS
Director of Cell & Gene Therapy Unit
Alexandra currently leads OcyonBio’s Cell & Gene Therapy Unit, responsible for creating the next generation of cell therapy manufacturing processes and analytics, incorporating bleeding-edge technologies and scientific know-how to enable rapidly scalable and agile manufacturing of complex medicines.
As part of the pioneering team, she has been involved in the design & development of the vector facility we are building today, where we’ll be generating & supplying the raw materials to support the development of novel cell & gene therapies. The area focuses on both: Blood processing & immune cell banking, and Vector Manufacturing offering AAV, Retro- & Lentiviral vector platform production capabilities.
Prior to joining OcyonBio, she broke into the CGT space by joining the CytoImmune Therapeutics team as Vector Development Scientist. Where she led process development efforts for CAR-NK Immunotherapy program enabling a successful tech transfer from discovery lab in CA into their manufacturing facility in PR in short of just 6 months. These efforts accelerated manufacturing activities to begin within the year of opening the facility.

Marvin Mercado
Director of Technology Transfer and Process Support

Marvin Mercado
Director of Technology Transfer and Process Support
Marvin Mercado, a chemical engineer with a passion for innovation and process optimization.
With a B.S. in Chemical Engineering from the University of Puerto Rico at Mayagüez, a Master’s degree from Iowa State University, and over 15 years of combined academic and industry experience, Marvin has a deep understanding of the biopharma field. As an expert in both upstream and downstream process development, he is well-versed in manufacturing process design and optimization as well as facility design, specifically in the areas of monoclonal antibodies, bi-specific antibodies, and antibody drug conjugates.
Marvin has a proven track record of success working with start-ups in the high-tech hubs of Austin, TX and the San Francisco Bay Area, bringing his expertise and passion to drive results.
Natalia Zayas Godoy
Legal Counsel

Natalia Zayas Godoy
Legal Counsel
She was the Associate Attorney at Coto & Associates. Ms. Zayas Godoy’s professional experience is in the private practice sector and she has worked in bankruptcy, health insurance, taxes, and trademarks areas. She has been involved in issuing legal opinions to clients and prospective clients, on renewal processes and declaration of continued use, declarations of first use, change of owner, change of address, and registration of trademarks applications.
Natalia obtained her B.A. in Human Resources, Magna Cum Laude from the University of Puerto Rico, in 2011, and her J.D. from Inter American University School of Law in 2014. She is admitted to the bar of the Commonwealth of Puerto Rico and is a member of the Colegio de Abogados de Puerto Rico.
William Rodríguez
Senior Project Manager

William Rodríguez
Senior Project Manager
William is the Project Manager at Biosciences Corp. He has over 28 years of industry experience with large to small multi-discipline projects ranging from Cell & Gene Therapy Suites, pharmaceutical, maximum security prisons to high rise buildings in all construction phases. Deep experience as project manager of major construction projects in Puerto Rico. Worked with major international general contractors and with local contractors in projects on the island. Highly experienced in cost control, estimating, design coordination, and scheduling.
Saddy Rivera
Senior Manager, Microbiology laboratory

Saddy Rivera
Senior Manager, Microbiology Laboratory
Saddy is the Senior Manager for the Microbiology laboratory at OcyonBio with 25+ years experience on Quality and Compliance for national and international pharmaceuticals, biologics, and medical device companies. She brings in-depth knowledge and experience in the biopharmaceutical industry with extensive experience in Microbiology, Quality Assurance, Environmental Monitoring, and Aseptic practices, supporting New Product introduction / pre-approval inspections, among other activities.
Janitza Arizmendi
VP of HR and Scientific Support Group

Janitza Arizmendi
VP of HR and Scientific Support Group
Human Resources Leader with over 15 years of experience within pharmaceutical, healthcare, and electronic manufacturing industry.
During her tenure within the pharmaceutical industry, has served as a HR Champion for the start-up and Commercialization of the First Vial Filling line using Isolation Technology, for aseptic filling of liquid and lyophilized vials. Supporting seamless technology and product transfers for over five key Biologics drug product facility and over two hundred key product transfers, encompassing major site expansion and workforce cultural change.
Outstanding track record of achievements in the pharmaceutical arena , driven by continuous development and enhancement of systems, procedures, processes , and workforce development, with proven ability to develop and manage relationships with multi- cultural, complex organizational client based on sound, decisive HR leadership.
Ricardo Zayas
COO and Operational Lead

Ricardo Zayas
COO and Operational Lead
He has 30+ years of experience in Pharmaceutical Manufacturing. He has extensive expertise in global and local pharma, operations management and supply chain. He is an expert in turning operations around and has a strong understanding and experience of global pharmaceutical markets. He is the Senior VP of Operations at AMAG Pharmaceuticals. He is also the Senior VP of global manufacturing operations and supply chain at Bristol Myers Squibb and Executive VP of Operations at Romark Pharmaceuticals.
Kevin Colón
Senior Services Manager

Kevin Colón
Senior Services Manager
He is the Principal Scientist at Biosciences Corp LLC and a Scientist at FibroGen, Inc. He is also a Research Scientist at View Point Therapeutics, Inc. He has experience in cell culture, stable cell line generation, and molecular biology techniques (DNA/RNA/protein). He has engineered multiple suspension and adherent cancer cell lines for oncology drug candidates screening using cutting-edge CRISPR technology. He has 10+ years of wet laboratory experience in molecular biology, cell biology, pharmacology, and several medium-to-large animal model systems.
Gabriel Gracia Maldonado
Operations Manager

Gabriel Gracia Maldonado
Operations Manager
Gabriel Gracia Maldonado is the Operation Manager at OcyonBio. He has a PhD degree in Pathobiology and Molecular Medicine with specialty in Heme/Onc. He was the Principal Scientist at Kurome Therapeutics, where he led their in-vitro and in-vivo efforts for drug candidate screening. He has experience in cell culture, flow cytometry and molecular biology techniques (DNA/RNA/Protein). He has vast experience in engineering and characterizing multiple suspension and adherent cancer cell lines for oncology drug candidate screening. He has 10+ years of wet laboratory experience in molecular and cellular biology, pharmacology, and mouse model systems.
Celeste Miranda
Senior Project Manager, PMO

Celeste Miranda
Senior Project Manager, PMO
Celeste is member of Global PMO, responsible for managing complex life sciences projects, including the Tech Transfer project for Biosimilar Solutions. In this role, she will manage design and construction projects for both landlord and tenant clients (ranging from start-up biotech’s to large pharma), interfacing directly with clients to define project requirements, scope of work, project delivery resource requirements, cost estimate & budget, cash flow, work plan schedule & milestones, quality control, and risk identification. Celeste also leads and manages the execution of our Project Based Organization.
Celeste initiated her career at Amgen in Juncos, PR, working as a Manufacturing Associate Engineer in the Syringe and Filling area at AML14. She later joined Bristol Myers Squibb, Syracuse, NY as a Validation Engineer. During that time, she was responsible for delivering validation projects arising from change controls, capital projects, shutdown, and revalidation program, under strict deadlines to ensure customer success. Prior to joining Ocyonbio, Celeste held a position as a Manager, Project Manager: Operational Excellence PMO at Bristol Myers Squibb NY. In that role, she led the planning and execution of Site P1/P2 projects, which included strategic run-the-business initiatives and process/productivity improvements within Manufacturing Operations, Material Operations, Quality Control, and IT. She developed business case and project charters, define scope and resource requirements, and led all project activities and recurring status meetings to achieve the completion of key milestones. She also facilitated decision-making and conflict resolution while proactively identifying and escalating issues to the appropriate cross-functional team. Celeste holds a Bachelor of Science in Bioengineering from Syracuse University, College of Engineering and Computer Science, New York.
Dennisse Morales
Senior Manager

Dennisse Morales
Senior Manager for Parenteral Manufacturing and Tech Transfer
Dennisse is our Senior Manager for Parenteral Manufacturing and Technology Transfer at OcyonBio. She brings in her in-depth knowledge and experience within the biopharmaceutical space, with extensive experience in regulatory compliance for the implementation and improvement of automated and semi-automated Production and Process Controls (P&PC), leading the coordination, execution, and supervision of new products introduction and manufacturing activities amongst other critical tasks. Devoted in ensuring the highest standards of quality on both regulatory and internal benchmarks through organizational leadership, she initiated her career as a Project Engineer Leader with a combined experience achieved in Project Management, Business Development Strategist, Direct Sales, Manufacturing Engineering, Operational Infrastructure, Qualification and Validation activities, Quality Assurance, Materials and Logistics.










