
Solutions for the Challenges of Maintaining Viral Vector Stability & Quality through Process Development
Viral vectors, which acts as containers of a cell’s modified genetic material, were first used in research to transfer or alter genetic material in cells. With the wave of gene therapy, these vectors are being used more frequently in new therapies used to treat rare diseases. Due to a better understanding of gene therapy and the COVID-19 pandemic, viral vectors are currently being used for more common diseases and vaccines, implying a higher demand for the use of viral vectors and the need to reduce the costs of viral vectors.
As of December 2022, the FDA has approved nine gene therapies and two cell-based gene therapies. The rapidly growing gene therapy industry has an unmet demand in viral vectors due to issues related to production scale, cross-contamination, quality, regulatory requirements, and supply chain.
- Manufacturing Scale-Ups:
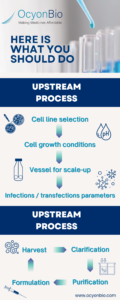
The production of viral vectors in the industry is not regulated, meaning each company has its own process development to produce viral vectors. Problems arise when companies try to scale up from clinical trials to production scale and need more knowledgeable people to fix them. To scale up, companies must be consistent with cGMP practices for a quality product. There are many variations in process development in manufacturing high-quality products. In the upstream process, variations in production cell lines, vectors, etc., can affect the results of scale-up production. Upstream process development for viral vectors should include cell line selection, cell growth conditions, scale-up vessels, and infection or transfection parameters. There are many risks to the product at these stages of the upstream process alone. Other conditions, such as temperature, pH, nutrition, osmotic pressure, etc., can affect the quality, stability, and quantity of the viral vectors.
On the other hand, downstream processes include harvesting, clarification, purification, and formulation. Problems with downstream processing in the viral vector space stem from the need for innovative technologies for clarification and purification. Because viral vectors are complex macromolecules, they require different purification methods than traditional chromatography for proteins. Conventional chromatography, which separates solid and liquid mixtures of a specific size, has become obsolete for viral vectors due to their larger size than other biological agents. Therefore, it is more appropriate to use a membrane for purification due to the membrane’s pore size.
- Cross-contamination:
When cross-contamination occurs, it can affect the entire development process of a product, from development to shipment. Cross-contamination in a manufacturing facility can come from people, raw materials, equipment, and the air. According to Sandra Acosta from EG Life Sciences, facility design must adequately control air pollution, inflow and outflow of raw materials, and people’s movement.
- Quality:
A significant issue in viral vector production is maintaining vector yield. Therefore, another major challenge is maintaining product quality. However, if aggregation occurs, the quality of the viral vector may be compromised, resulting in a loss of potency. The pH and ionic strength of the carrier formulation are also known to affect carrier stability. (Floyd & Sharp, 1979). Meanwhile, viral vectors are more complex molecules than recombinant and other proteins, which means that new quality tests must be created and validated for each viral vector. This step can delay the product due to the need for validated standards, leading to more trial and error in those standards (Van Der Loo & Wright, 2015).
- Regulatory requirements:
With the advent of viral vectors in the biopharmaceutical industry, specific regulations must be implemented to provide quality products to customers. We adhere to the cGMP regulatory requirements of the FDA, EMA, and other agencies when manufacturing viral vectors. Establishing cGMP for early-stage manufacturing can take time due to the different regulatory requirements required at each stage.
- Supply chain:
Once the viral vectors are manufactured and meet quality and all regulatory requirements, they must be shipped. Another area for improvement with viral vectors is maintaining their viability during transportation and storage. One method t to improve viral vectors viability and quality was formulated by MA Croyle, Division of Pharmaceutics at the University of Texas at Austin College of Pharmacy, who developed various buffer systems to store viral vectors alongside known cryoprotectant substances.
In the future of viral vectors, increased FDA and EMA approvals for gene therapy and clinical trials will increase the demand for viral vectors. This growing demand creates some of the manufacturing issues mentioned above.
At OcyonBio, we drive your vision by offering up to 150,000 sq. ft. of cGMP space to address these viral vector bottlenecks. Our space can be customized to meet your needs from clinical to commercial production. At OcyonBio, we rely on well-trained and well-prepared personnel to meet your needs, from process development technology transfer to manufacturing, quality control, and more. Our quality control services rely on a comprehensive in-house QC laboratory, facility and operations control support, and support throughout the product’s life cycle. We can also assist you in formulating compliance control strategies for your products, such as planning and collection, product delivery, transportation, and production. Our goal is co-creating the future by delivering quality and accessible medicines.
Learn more at: Capabilities & Services – OcyonBio
In collaboration by: Tatiana P. Serrano-Zayas – COOP Student, University of Puerto Rico at Mayaguez
Edited by: Ashley Cortés, Marketing Specialist at OcyonBio
References:
Acosta, S. (n.d.). Viral Controls and Prevention of Cross-Contamination in the Vaccine Industry. https://blog.eglifesciences.com/viral-controls-and-prevention-of-cross-contamination-in-the-vaccine-industry
Capra, E., Gennari, A., Loche, A., & Temps, C. (2022, March 31). Viral-vector therapies at scale: Today’s challenges and future opportunities. McKinsey & Company. https://www.mckinsey.com/industries/life-sciences/our-insights/viral-vector-therapies-at-scale-todays-challenges-and-future-opportunities
Croyle, M., Cheng, X., & Wilson, J. (2001). Development of formulations that enhance physical stability of viral vectors for gene therapy. Gene Therapy, 8(17), 1281–1290. https://doi.org/10.1038/sj.gt.3301527
Desai, A. (n.d.). Challenges in upstream bioprocess development for viral vector production. BioInsights. https://insights.bio/immuno-oncology-insights/journal/article/190/challenges-in-upstream-bioprocess-development-for-viral-vector-production
Floyd, R., & Sharp, D. G. (1979). Viral aggregation: buffer effects in the aggregation of poliovirus and reovirus at low and high pH. Applied and Environmental Microbiology, 38(3), 395–401. https://doi.org/10.1128/aem.38.3.395-401.1979
Just a moment. . . (n.d.). https://www.sciencedirect.com/science/article/abs/pii/S221133982100112X
Lacouchie, J. (2021, May 10). Scaling up viral vector production: the importance of quality. BioInsights. https://www.insights.bio/cell-and-gene-therapy-insights/journal/article/2199/Scaling-up-viral-vector-production-the-importance-of-quality
Singh, N., & Heldt, C. L. (2022). Challenges in downstream purification of gene therapy viral vectors. Current Opinion in Chemical Engineering, 35, 100780. https://doi.org/10.1016/j.coche.2021.100780
T. (2022, January 17). Viral vector and vaccine development: beware the pitfalls of scale-up. Batavia Biosciences. https://www.bataviabiosciences.com/viral-vector-vaccine-development-scale-up/
Van Der Loo, J. C., & Wright, J. F. (2015). Progress and challenges in viral vector manufacturing. Human Molecular Genetics, 25(R1), R42–R52. https://doi.org/10.1093/hmg/ddv451


