Overview
Our capabilities and resources enable CMC data to support regulatory applications, providing dedicated autonomous manufacturing capacity with interconnected infrastructure and systems to support phased appropriate development for early development, pre-clinical, clinical, and commercial start.
COLLECTION
- Shipping Kits & Labels Provided
- Initiate Chain of Custody, Identity & Condition
INBOUND
- Facilitate Air/Ground Transportation Services With Qualified and Trusted Partners
cGMP MANUFACTURING
- Process & Analytical Development
- Cell Select, Activate, Engineer & Expand
- Final Packaging
- Quality Release

OUTBOUND
- Facilitate Air/Ground Transportation Services With Qualified and Trusted Partners

TREATMENT
- Return to the Health Care Provider
Process, Analytical Development and MS&T
Development/Optimization (PD/AD)
- Optimize processes and analytical methods using innovative solutions
- Collect data to document process improvements, scale-up, comparability, and stage-appropriate CMC requirements
- Draft and execute study protocols and reports

Technology Transfer (MS&T/AD)
- Perform comprehensive evaluation of processes and supporting analytics
- Develop stage-appropriate Technology Transfer plans, write SOPs, reports, Batch Records, and test methods

cGMP Manufacturing Support (MSSupport&T)
- Provide process knowledge and support during Technology Transfer
- Train cGMP Manufacturing Operators on partner technology and process SOPs
- Monitor cGMP manufacturing campaigns in real time, evaluate root causes of ongoing process issues, provide overall support of cGMP manufacturing
Quality Control
Comprehensive In-house Quality Control Laboratories
- Testing of incoming raw materials, iPCs, and final product
- Core chemistry, analytical, and microbiology analysis (e.g., compendial analysis, ELISA, rapid product sterility, microbial identification, spectroscopy)
- Stability studies, management of reference standards and controls, and facilitating outsourced characterization testing as required
Support of Facility and Operational Control
- Facility controls via environmental, personnel, and utility monitoring
- QC labs are integrated with Operations, located adjacent to the cGMP Manufacturing cleanroom core
Supports the Complete Product Lifecycle
- QC supports the product lifecycle, from clinical through commercial production
- QC performs in house testing using rapid microbiological methods (sterility, endotoxin, mycoplasma) to ensure quick product understanding, especially important for autologous products
- Close partnership with MS&T for method transfer, optimization, and qualification/validation
Compliance Control Strategy

Scheduling & Collection
- Chain of Identity
- Chain of Custody
- Chain of Condition

Product Delivery
- Product Disposition
- Product Specifications
- QC Testing
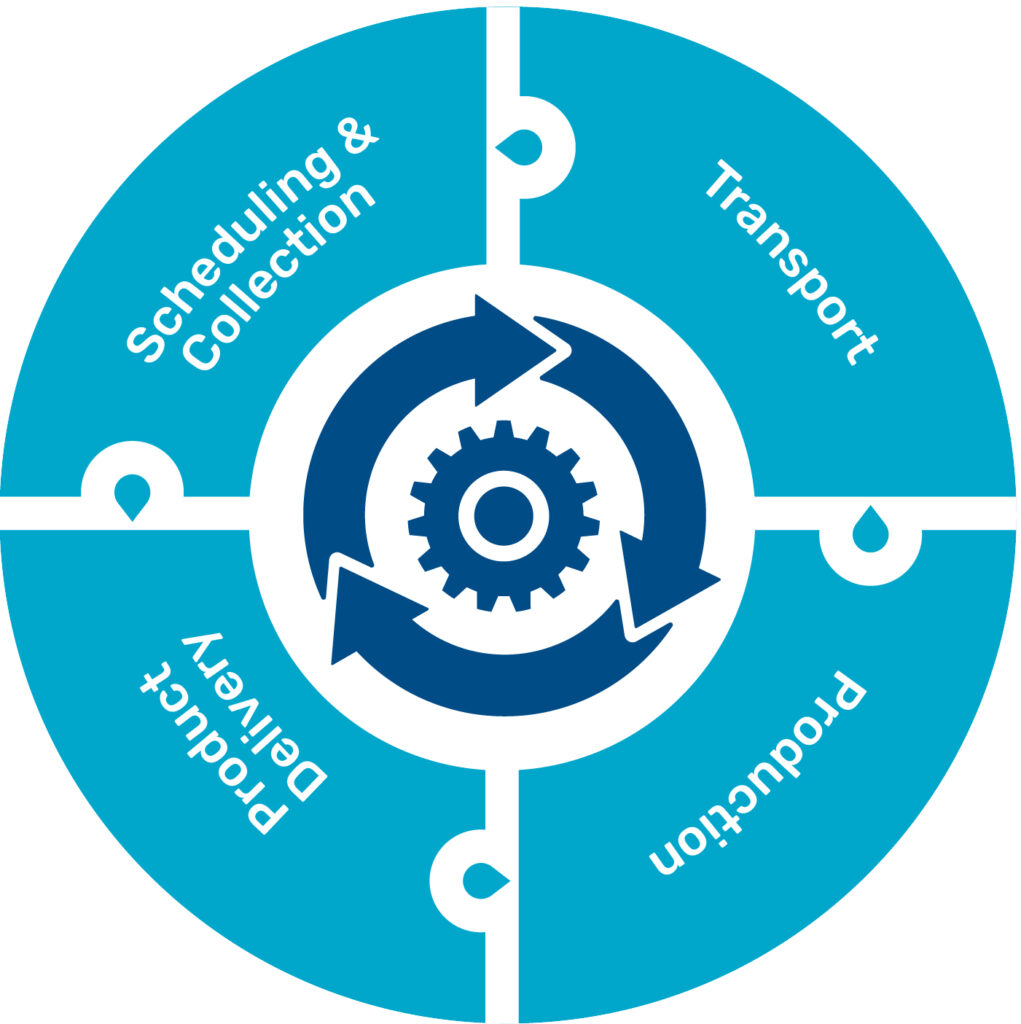

Transport
- Active Product Monitoring
- Incoming Product Integrity
- Supplier and Material Management

Production
- Training
- Facility Monitoring / EM
- EBR
- In-process Controls
- Plant Floor QA

